

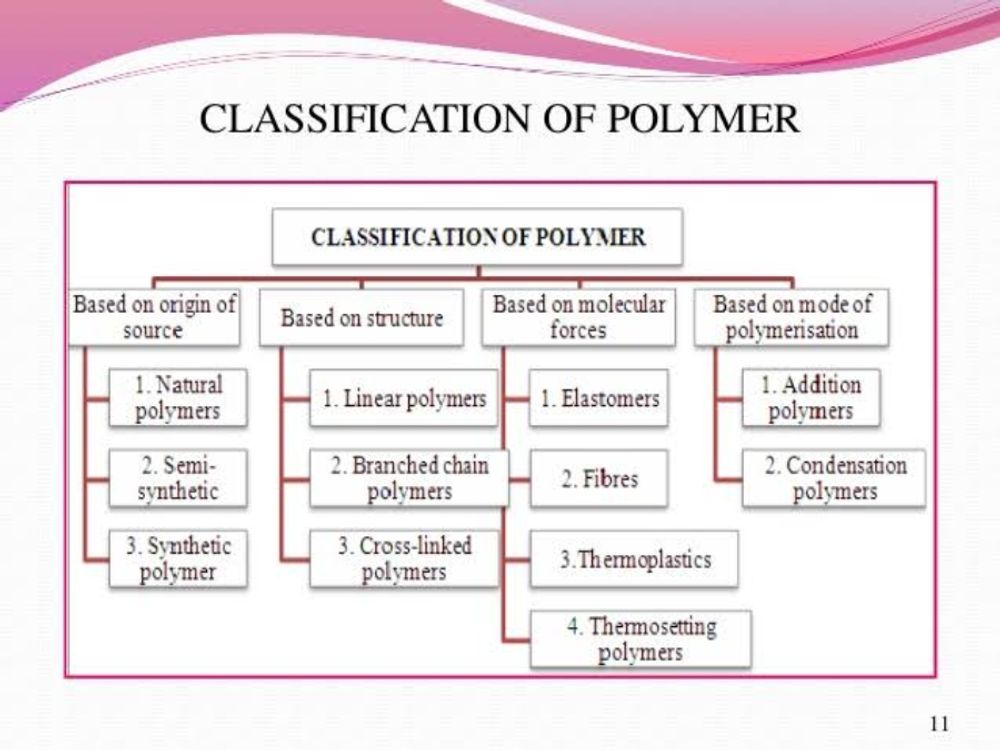
Based on the source of polymer they are classified into three sub categories.
Polymers which are isolated from natural origin are called natural polymers. These polymers are found in plants and animals. E.g. Proteins, cellulose, starch, some resins and rubber.
The polymers are obtained by chemical treatment of natural polymer to improve their physical properties are called semisynthetic polymers. Semisynthetic polymers are simply chemically modified natural polymers. E.g. Cellulose acetate (rayon) and cellulose nitrate from cellulose.
Polymers are synthesized in laboratory from monomers are called synthetic polymers. E.g. Polyethylene, Nylon 6,6, Buna–S, Polystyrene, etc.
On the basis of the polymers structure they are
divided into three subgroups.
These polymers consist of long and straight chains well packed structure. The examples are high density polythene, polyvinyl chloride, etc. They have high melting point, high densities and high tensile strength.
These polymers contain linear chains having some branches and irregular packed structure.
e.g., low density polythene and glycogen. They have low tensile strength, low density and lower melting point in comparison with linear polymers.
These are usually formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains. They are hard and rigid. E.g. Bakelite,melamine, etc.
Addition Polymerization
• This is also called as chain growth polymerization.
• In this, small monomer units joined to form a giant polymer.
• In each step length of chain increases. Condensation Polymerization
• In this type small molecules like H2O, CO, NH3 are eliminated during polymerization (step growth polymerization).
• Generally, organic compounds containing bifunctional groups such as idols, -dials, diamines, dicarboxylic acids undergo this type of polymerization reaction. For example, Preparation of nylon-6, 6.
Glycogen is a readily mobilized storage form of glucose. It is a very large, branched polymer of glucose residues that can be broken down to yield glucose molecules when energy is needed.
Olefins, also known as alkenes, are unsaturated hydrocarbons containing one ore more carbon–carbon double bonds. Formula: CnH2n
Elastomers are also known as rubbers. These are solids with elastic properties. In these elastomeric polymers, the polymer chains are held together by the weakest intermolecular forces. These weak binding forces permit the polymer to be stretched. A few crosslinks are introduced in between the chains, which help the polymer to retract to its original position after the force is released as in vulcanized rubber. These are polymers which can undergo large elongations under load, at room temperature, and return to their original shape when the load is released.
E g : una-S, buna-N, neoprene, etc. There are number of man-made elastomers in addition to natural rubber.
Fibers are the thread forming solids which possess high tensile strength and high modulus. These characteristics can be attributed to the strong intermolecular forces like hydrogen bonding. These strong forces also lead to close packing of chains and thus impart crystalline nature.
Eg: Nylon 6, 6, Terylene, etc.
Some polymers soften on heating and can be converted into any shape that they can retain on cooling. The process of heating, reshaping and retaining the same on cooling can be repeated several times. Such polymers that soften on heating and stiffen on cooling are termed thermoplastics.
Eg: Polyethylene, PVC, etc.
Some polymers on the other hand under go some chemical change on heating and converted themselves into an infusible mass, and once set cannot be reshaped. Such polymers that become an infusible and insoluble mass on heating are called thermosetting polymers.
Eg: Epoxy Resin, polyester, etc








