
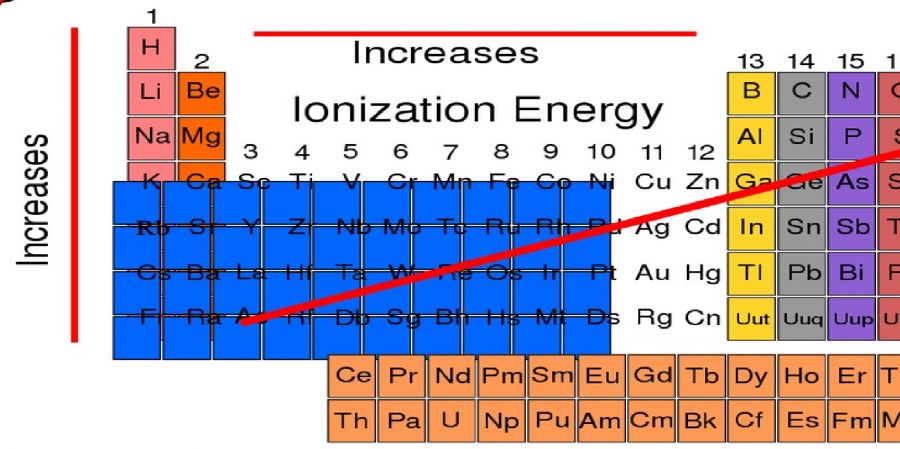
Ionization energy is a measure of the energy of an element entering a chemical reaction that requires ion formation or electron loss. It is also commonly associated with genetically engineered computer compounds. Ionization Energy, also known as ionization Energy, is a property of all elements in the periodic table. What does the ion potential mean? This is the amount of ENERGY required to release an electron from the neutral atoms that form the ion. Therefore, it is tightly bound (difficult to remove). Based on these two principles, francium is the simplest ioni, and the force of ionize measures the bias of the neutral atom to withstand the loss of electrons. For example, it takes a lot of ENERGY to remove electrons from neutral fluorine atoms and form fully charged ions. F (g) F + (g) + e Ho = 1681.0 kJ / mol. Therefore, the initial of that potential of helium is high and the resolution of francium is very low. y, the initial of that factor of the required ene. Removal of external or higher electron from neutral atoms in the gas phase. A low ionizing force means that very little energy is needed to remove the external electrons. This is due to the increase in nuclear charge, which causes external electron to bond tightly to the nucleus. That of a chemical type (that is, an atom or molecule) is the that required to extract an ELECTRON from a gas or ion-atom. This range, also called the ionizationpotential, is measured in volts. In chemistry, it usually refers to a single molecule (molar ionization energy or enthalpy) and is expressed in kJ / mol. In atomic physics, of that power is usually measured in electro volt (eV) units. The larger the atom or molecule, the lower the that , but the smaller the molecule, the higher the that tends to be. The ionization's potentials depends on the atomic orbital and molecule depending on the electron's. In general, the nth ionzing force is the force required to extract the nth eletron after the emission of the first n1 eletron. It is considered a measure of the tendency of an atom or ion to provide an electron or electron binding force. The stronger the of that, the more difficult it is to remove the ELECTRONS. It can indicate the return of the element. Hypoionized ions are drugs that are usually formed by reducing cations and binding to their salts to form salts.




